Chimeric Therapeutics is a drug development company focused on novel CAR-T cell therapies for solid tumors
With a successful $4m seed round completed in September and favourable results from pre-clinical studies of CTLX-CAR T, Chimeric Therapeutics is gearing up for an exciting year ahead by listing in early January 2021 and commencing their highly-anticipated Phase 1 clinical trials.
CTLX-CAR T has shown early signs of halting the rapid progression of Glioblastoma Multiforme (GBM), empowering the patient’s immune system to fight the cancer. GBM is an aggressive type of tumor affecting the brain (or spinal cord) which is often fatal within 12-14 months.
These new trials will no-doubt attract considerable attention, with many big pharma companies making significant investments at pre-clinical stages. Given this, it’s reasonable to assume it’s only a matter of time before Chimeric enters similar discussions.
What excites us, is the involvement of Paul Hopper, a highly respected and proven biotech entrepreneur, supported by an exceptional board and management team.
Some key highlights
- Phase 1 GBM clinical trial underway with two patients already treated. First data expected in Q1/Q2 2021.
- Clinical trial run by leading US cancer institute, City of Hope Cancer Centre in Los Angeles (one of the leading cancer hospitals in the US).
- Strategy to move directly into a 50 –75 patient Pivotal Study following a successful Phase 1 clinical trial.
- Established safety profile (prior use in humans as an imaging agent).
- Robust and unambiguous intellectual property profile with long life (composition of matter patent expires 2036).
- Significant drug administration advantages: patients are treated with the drug as outpatients, therefore not requiring hospitalisation.
______
Board and key management
Executive Chairman: Paul Hopper is the founder of Chimeric and has over 25 years of experience in the medical, healthcare, and life sciences sectors. Focused on start-up and rapid growth companies, he has served as either Founder, Chairman, Non-Executive Director, or Chief Executive Officer of more than fourteen companies in the US, Australia and Asia. Previous and current Boards include Viralytics (ASX: VLA), Imugene (ASX: IMU), pSivida (ASX: PSD), Polynoma (wholly owned subsidiary of HKG:0775), Somnomed (ASX: SOM), Suda (ASX: SUD), C19 Therapeutics Pty Ltd, and Prescient Therapeutics (ASX: PTX).
Non-Executive Director: Leslie Chong has more than 21 years of oncology experience with comprehensive clinical development experience in global Phase I-III studies from start-up to registration. Ex Senior Clinical Program Lead at Genentech, one of the world’s most successful biotech businesses developing therapies across all cancer indications. Previously at global majors GlaxoSmithKline (NYSE: GSK) and Exelixis (NASDAQ: NEXEL) in cancer therapy development.
Non-Executive Director: Dr Lesley Russell has more than 25 years of senior international operational and leadership experience having worked at Amgen (NASDAQ: AMGN), Eli Lilly (NYSE: LLY), Cephalon (NASDAQ: CEPH), and Teva (NYSE: TEVA). As Chief Medical Officer and Chief Operating Officer, Dr Russell has wide experience in the therapeutic areas of hematology, oncology, neurology, psychiatry, pain and inflammation, respiratory medicine, and stem cell therapy. Dr Russell has extensive knowledge and experience with new drug development along with CAR T therapies.
Chief Operating Officer: Until recently Ms Jennifer Chow was Head of Global Marketing, Analytics, and Commercial Operations at leading global CAR T company Kite Pharmaceuticals (acquired by Gilead Sciences in 2017 for US$11.9bn).
Chief Medical Officer: Until recently, Dr Syed Rizvi, was the Chief Medical Officer at CAR T leader, Legend Biotech, which was the largest biotech listing on NASDAQ this year.
______
Clinical Development Plan: Timeline to fast-track New Drug Application (NDA
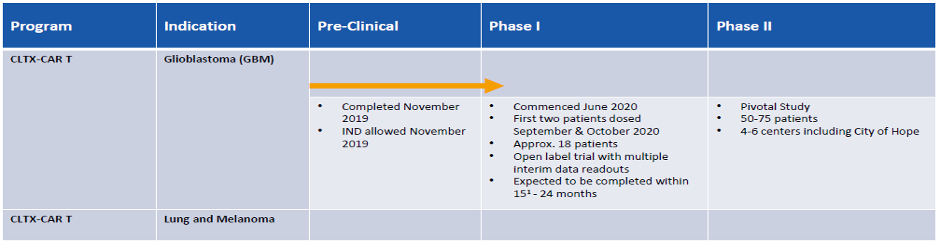
Throughout the 15-24 months patient’s results will be staggered. Thus far, two patients have received treatment with brain scans to be conducted four weeks after last administration. Early indications are good, with results expected soon. Given the rarity of this disease, and the non-existence of any effective treatments, the FDA is more likely to expedite its application improving the chances for early commercial treatment. A comparison being Avastin®, approved for brain cancer reported to have minimal impact, however, still administered as patients would rather have something over nothing. Given this, Chimeric will be seeking early feedback from the FDA for approval based on Phase 2 trials being in line with Phase 1.
Recent notable CAR T transactions

- Legend Biotech: Received bid for $1b on the book, accepting $424m (largest biotech IPO this year).
- Gilead acquired Kite Pharma for $11b.
- Celgene acquired Juno for $9b.
- October 2020 company IN8Bio bio are raising $85m for CAR T phase 1 trial.
Keeping in mind these are all pre-clinical studies, it is worth noting, as it increases the probability of Chimeric following suit.
______
What’s next?
Baker Young is excited to be working with and supporting Paul Hopper and his team, given the attention CAR T treatment is receiving on a global scale. Having witnessed significant interest from big pharma, evidenced by multiple large transactions, it’s certainly an opportune time to be associated with a drug that has the potential to be the catalyst for a significant breakthrough in the treatment of GBM.
While the offer has closed, you can learn much more about the company and its research through the prospectus which you can read here.
General Disclaimer
This article has been prepared for the general information of investors and not having regard to any particular person’s financial situation, objectives, or needs. Accordingly, in so far as any information may constitute advice (whether express or implied), it is general advice and no recipient should rely upon it without having obtained specific advice from their advisor at Baker Young Limited. Baker Young Limited makes no representation, gives no warranty, and does not accept any responsibility for the accuracy or completeness of any recommendation, information, or advice contained herein. To the extent permitted by law, Baker Young Limited will not be liable to the recipient or any other persons in contract, in tort or otherwise for any loss or damage (including indirect or consequential loss) as a result of the recipient, or any other person acting or refraining from acting in reliance on any recommendation, information or advice herein. Baker Young Limited or persons associated with it may have an interest in the securities or financial products mentioned in this document and may earn brokerage and other fees as a result of transactions in any such securities. Australian Financial Services Number 246735.
