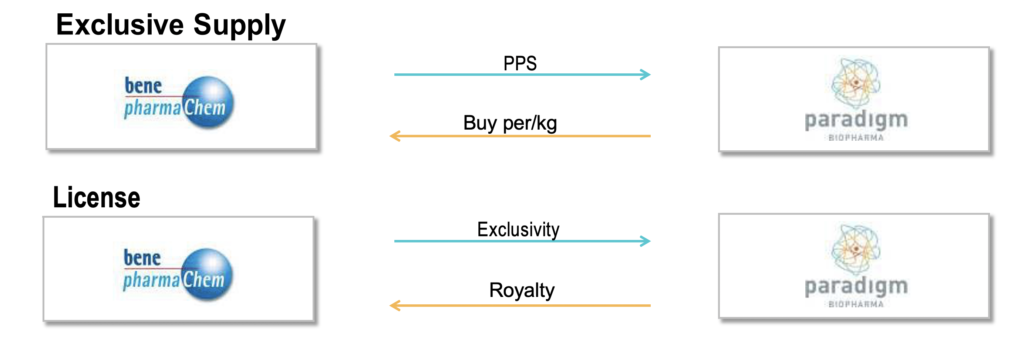
In recent weeks, Paradigm has achieved additional key milestones that strengthen the company’s position. The most anticipated being the amendment to its exclusive licence and supply agreement with bene pharmaChem. The revised terms under this agreement are pivotal for ensuring the long term success of Paradigm and its ability to execute not only on Osteoarthritis (OA) treatment but the many other applications the company holds patents for.
Understanding why this matters
Bene pharmaChem owns the only drug master file (DMF) with the FDA to manufacture iPPS for human applications. Any alterations in one of the many stages of production, such as the quality of the charcoal, beechwood chips, or temperatures, can alter the final product and drug molecule creating headaches for competitors who struggle to replicate PPS to a standard that is accepted by the Food and Drug Administration (FDA). If the end molecule doesn’t match the bene pharmaChem approved PPS on file with the FDA, it cannot be classed as PPS (not a bio-equivalent).
Due to these complexities, as long as Paradigm remains an exclusive customer of bene pharmaChem, it is highly unlikely competing products can penetrate Paradigm’s market position – it would take years for an alternative PPS manufacturer to compete given the strict regulatory process any new drug needs to endure to be deemed safe.
Several attempts to replicate PPS have been made by some of the largest pharmaceutical companies. All have outlaid significant capital and failed. These companies have reverted to being customers of bene pharmaChem.
Why so confident? A real-world example…
Elmiron by Jensen (a subsidiary of Johnson and Johnson (J&J))
Bene pharmaChem has a long-standing exclusive agreement with J&J for the supply of PPS which is used to treat a rare orphan condition relating to inflammatory bladder syndrome (interstitial cystitis or IC). Bene pharmaChem has honoured this agreement as the sole provider of PPS since 1996. Remarkably there has been no generic form of Elmiron developed despite corresponding patents having expired in 2010. This is a testament to the loyalty of bene pharmaChem, as well as highlighting the difficulty in replicating PPS. As mentioned, bene pharmaChem remains the only recognised FDA approved supplier of PPS. This has enabled J&J to increase the price of Elmiron, which would not be possible if a generic version was available.
Fortuitously, Paradigm is in a similar position. The company is placed to service a market where no competing FDA PPS molecules are approved. Given the complexities involved in replicating bene pharmaChem PPS, if phase three trials are successful, it is reasonable to expect Zilosul® (iPPS approved trademark) to have exceptional pricing power, similar to Elmiron.
This is something that can’t be overlooked, as it gives Paradigm first-mover advantage if further attempts to replicate a PPS bio-equivalent fail. Additionally, it would make no sense for bene pharmaChem to supply competing companies access to PPS for OA. It would be to their detriment given Paradigm’s exclusive supply agreement. The absence of a generic offering would enable Paradigm to benefit from favourable margins, with no pressure on pricing. Given bene pharmaChem receives a 2% royalty on sales, it is undoubtedly within their interests to act in good faith throughout the duration of the agreement.
Who are bene pharmaChem ?
Bene pharmaChem is a family-run private business that has manufactured PPS since the 1940s. Bene pharmaChem operates from four production sites and is manufactured in a state-of-the-art facility with the primary purpose of ensuring PPS is manufactured to reliable and consistent quality. Equipment used to produce PPS has been custom-built by bene pharmaChem using in-house IP spanning generations. Given the bespoke nature of such equipment and technology, this significantly strengthens the integrity of PPS. Bene pharmaChem employs 175 staff and has excess capacity to handle any additional demand. Bene pharmaChem is audited frequently by regulators, and the FDA, as well as major customers, ensuring the highest quality product is maintained.
SAS results continue to impress
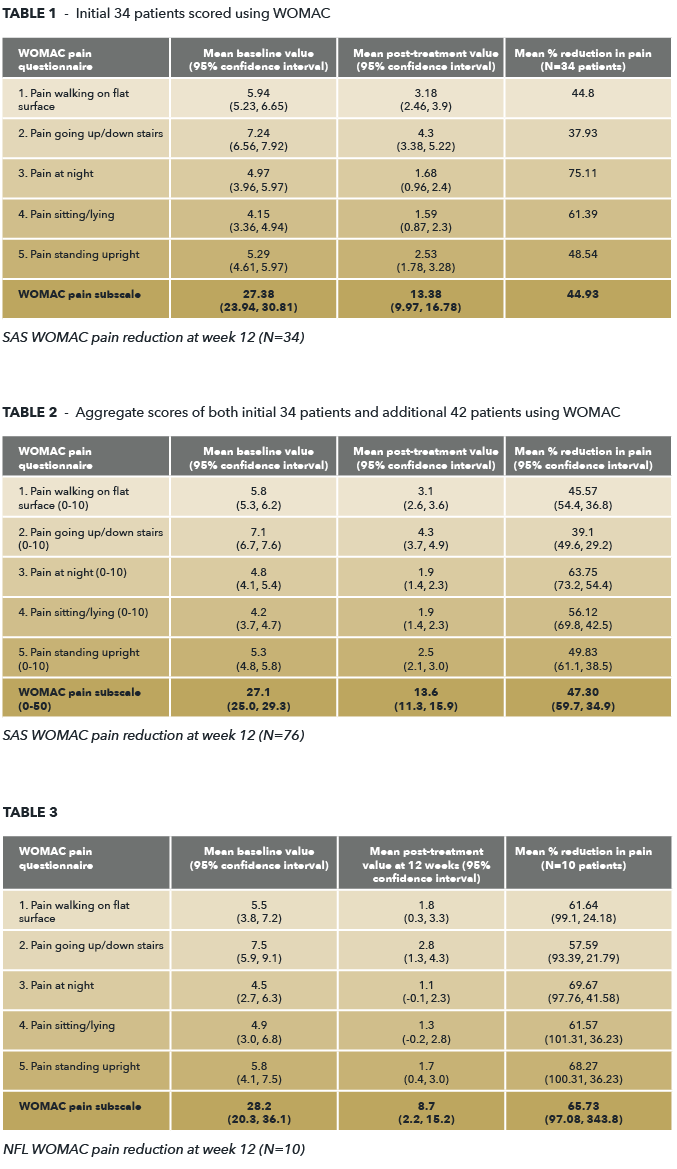
Therapeutic Goods Administration (TGA) Special Access Scheme (SAS) Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores continue to return positive results in line with expectations. Pain reduction in 76 SAS patients (42 new patient data), showed that the phase 3 iPPS formulation was well tolerated and demonstrated a continued excellent safety profile, with no serious adverse events reported. These results provide important Real World Evidence (RWE) illustrating anticipated responses in everyday clinical practice supporting Paradigm’s Phase 3 clinical trial. We expect the company to continue to report on a cohort of 100 SAS results in the months ahead. It is important to note, all patients treated prior to the WOMAC pain and function subscales were assessed using Knee Injury Osteoarthritis Outcome Score (KOOS). As can be seen below in the tables provided 1, 2 & 3 WOMAC results for all 76 SAS patients and NFL Expanded Access Scheme (EAS) patients (n=10) continue to illustrate pain reduction and improved mobility results in line with KOOS, if not better. This bodes extremely well leading into Phase 3 trials.
Understanding the results
Mean Reduction in pain improved in table 2, as opposed to table 1 indicating, on aggregate, the recent round of SAS results (n=42) was an improvement. These are very good results given the absence of adverse effects.
The icing on the cake… the European Medicines Agency (EMA)
Approval by the EMA represents a key milestone for Paradigm allowing applications for clinical trials to begin in the European Union. This achievement clears the way for product registration and commercialisation plans for Zilosul®. Paradigm will submit to the FDA in coming weeks their Type C briefing book which will include the Phase 3 protocol as agreed upon with the EMA. Whilst nothing is certain, the recent milestone achieved with the EMA once again bodes well for a favourable outcome from the FDA for Phase 3 approval.
Overall
Paradigm continues to perform in line with expectations. The above market updates reflect a crucial time for the company. What is most pleasing, Paul Rennie (CEO and interim Chair) and his supporting management team continue to work tirelessly, ensuring the correct checks and balances are in place, allowing for a smooth transition through the many regulatory requirements needed to successfully execute on a product that we believe has the makings of a blockbuster drug. We remain supportive of Paul and his team and look forward to seeing how things progress in the coming months.
