Baker Young Corporate Advisory led the oversubscribed Entitlement Offer and Placement for SUDA Pharmaceuticals
last August in addition to the successful Placement in December 2020 (for which it received fees).
SUDA Pharmaceuticals Ltd (ASX:SUD) is one of the quiet achievers in the biotech sector that have, until recently, stayed off the radar of most investors, but that’s about to change.
The company works at the leading edge of the biotech/pharmaceutical market with its patented oral spray delivery process for drugs, with projects in the areas of migraines, short-term insomnia, and oncology. However, Managing Director, Dr Michael Baker, says the company is now set to acquire additional intellectual property in the areas of oncology and the central nervous system.
A core focus for the company is its OroMist® drug delivery technology that replaces the standard pill/capsule/injection delivery methods. It reformulates existing drugs and utilises a more effective and user-friendly oral spray delivery. Once sprayed into the mouth, the drug can gain direct access to the bloodstream by crossing the oral mucosa (lining of the mouth), as opposed to a pill that can lose potency and speed due to the reactive actions of stomach acids and breakdown by the liver. The OroMist® delivery process is particularly beneficial for patients who have difficulty in receiving medication by removing the need for injection, or requirement to swallow or inhale.
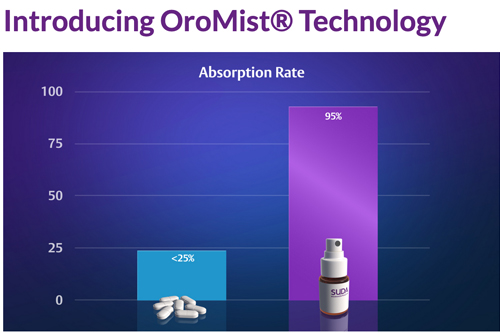
In addition, the company has received Therapeutic Goods Administration (TGA) approval for its treatment for short-term insomnia, ZolpiMist™, and it is currently working with Strides Pharma to reformulate the anti-migraine drug, Sumatriptan. SUDA also has a platelet reducing technology (anagrelide) that is formulated into an oral spray to limit the unwanted side effects on the heart which occur when the drug is delivered as a capsule due to the first-pass metabolism in the liver.
Additional innovative technologies by SUDA Pharmaceuticals
ZolpiMist™ is the company’s oral spray version of Sanofi’s blockbuster drug Ambien. Utilising the OroMist® technology for the drug’s administration induces sleep faster than delivery to the bloodstream by crossing the lining of the mouth, meaning it can work faster than the tablet. In July 2020, ZolpiMist™ received TGA approval for development here in Australia. Negotiations for an Australian partner are underway and the company has rest-of-world rights, ex-North America, for the commercialisation of the drug. Licence and supply agreements are in place with international drug companies Teva Pharmaceuticals, the world’s largest generics manufacturer, and Mitsubishi Tanabe Pharma Korea.
Sumatriptan, is the generic name for GlaxoSmithKline’s blockbuster migraine drug, known as IMITREX. The company’s development plan, including clinical trials, is fully funded by Strides Pharmaceuticals, one of India’s largest pharma companies. SUDA will supply the product to Strides, once it is commercialised.
Anagrelide, is the company’s move into the oncology field with a novel strategy for reducing the platelet counts in patients afflicted with cancer and thrombocytosis (elevated platelet levels). Many studies across numerous cancers demonstrate that when patients have elevated platelet levels, it results in a reduction in lifespan. Scientific research supports that platelets do play a role in assisting cancer cell growth and that they can shield cancer cells from our immune system. SUDA’s hypothesis is that using Anagrelide to lower platelets will help to increase cancer patient survival. Anagrelide has been in clinical use for more than 20 years, having received approval by the FDA and the EMA for the treatment of essential thrombocythemia. However, delivery of the drug as a capsule has unwanted side effects on the heart and an injectable formulation does not exist. Accordingly, SUDA’s oral spray delivery process is expected to provide the benefits of lowering cancer patient’s platelet levels without the damaging side effects on the heart. SUDA is excited to have a presence in this space as the market opportunity is quite large, with high platelet levels found in multiple cancer types such as small cell lung carcinoma, ovarian, bowel, renal, pancreatic, and breast cancers.
To lower the risks of development SUDA has recruited specialised skills from a leading global company, MedPharm, which develops drug delivery methods through the skin and cheeks. Early-stage testing by MedPharm has shown improved uptake of the drug and reduced negative side effects, identifying that SUDA’s oral spray application is potentially a safer route for the drug than pills.
With these exciting projects and the company’s proactive approach to new opportunities, Dr Baker says the company is in a unique position to address unmet needs in the drug delivery and oncology sectors.
SUDA’s Scientific Advisory Board is rich with international expertise, and the Board of Directors is led by industry stalwart and chairman, Paul Hopper, ably supported by industry professionals David Phillips, as Executive Director, and David Simmonds, in a non-executive Director role. At the helm is Dr Baker, whose experience prior to joining Suda was with Bioscience Managers. With a PhD in Biochemistry and an MBA from the University of Melbourne, he has a broad grasp of what is required to bring SUDA to a successful position in the Australian and international biotech marketplace.
About SUDA Pharmaceuticals Ltd
SUDA Pharmaceuticals Ltd (ASX: SUD) is a drug delivery company focused on oro-mucosal administration, headquartered in Perth, Western Australia. The Company is developing low-risk oral sprays using its OroMist® technology to reformulate existing pharmaceuticals. The many potential benefits of administering drugs through the oral mucosa (i.e. cheeks, tongue, gums, and palate) include ease of use, lower dosage, reduced side effects, and faster response time. SUDA’s product pipeline includes ZolpiMist™, a first-in-class oral spray of zolpidem tartrate for the treatment of short-term insomnia. ZolpiMist is approved by the TGA and is marketed in the USA. SUDA has rights to the product outside of the US and Canada. Other products in development include oral sprays for the treatment of migraine headaches, motion sickness, drug-resistant epilepsy, and certain cancers. For more information, visit www.sudapharma.com.
General Disclaimer
This article has been prepared for the general information of investors and not having regard to any particular person’s financial situation, objectives, or needs. Accordingly, in so far as any information may constitute advice (whether express or implied), it is general advice and no recipient should rely upon it without having obtained specific advice from their advisor at Baker Young Limited. Baker Young Limited makes no representation, gives no warranty, and does not accept any responsibility for the accuracy or completeness of any recommendation, information, or advice contained herein. To the extent permitted by law, Baker Young Limited will not be liable to the recipient or any other persons in contract, in tort or otherwise for any loss or damage (including indirect or consequential loss) as a result of the recipient, or any other person acting or refraining from acting in reliance on any recommendation, information or advice herein. Baker Young Limited or persons associated with it may have an interest in the securities or financial products mentioned in this document and may earn brokerage and other fees as a result of transactions in any such securities. Australian Financial Services Number 246735.
